Abstract
Chronic lymphocytic leukemia (CLL) is associated with increased numbers of T-cells that exhibit substantial alteration in composition and function. But their role in CLL pathogenesis remains largely elusive. On the one side, T-cells provide proliferation and pro-survival signals to CLL cells, but on the other side, they show features of activation and exhaustion, including overexpression of inhibitory molecules, like PD-1, suggesting their involvement in anti-tumoral activity. Since CLL T-cells retain the ability to produce cytokines and cytotoxic molecules, they were termed pseudo-exhausted. Results of previous studies were mostly based on blood samples of CLL patients, allowing only limited conclusions as interactions of immune cells and CLL cells mainly occur in lymphoid organs.
In the present study, we aimed at deciphering differences in phenotype, differentiation and functional status of T-cells in paired peripheral blood (PB) and lymph node (LN) samples of CLL patients, particularly focusing on T-cell exhaustion. We applied 12-color flow cytometry and ex vivo stimulation of primary CLL T-cells followed by intracellular cytokine and cytotoxic molecule detection. In addition, we quantified transcription factors that are known to regulate T-cell differentiation by intracellular flow cytometry.
In line with published data, we detected an increased proportion of CD45RO- CCR7- CD8+ effector T-cells as well as increased numbers of PD-1+ CD8+ T-cells in PB of CLL patients (n=22), compared to age-matched healthy individuals (n=19), and a positive correlation of these numbers with disease load. Despite of their enhanced expression of PD-1 and other inhibitory receptors, PB CD8+ T-cells from CLL patients exhibited increased production of granzyme B (GzmB), interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα) in comparison to cells from age-matched healthy donors, indicating disease-associated activation of PB CD8+ T-cells with intact functional capacity.
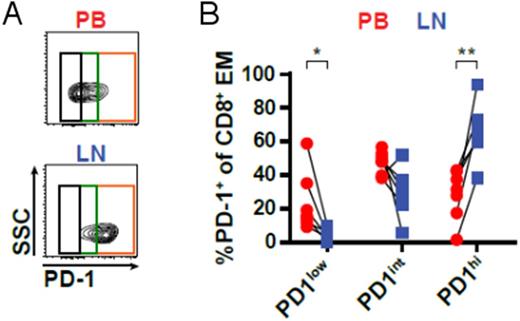
Subsequently, we compared CD8+ T-cells from paired LN and PB samples of CLL patients (n=7). This analysis revealed that LNs were enriched in CD45RO+ CCR7- effector memory T-cells (TEM cells) and contained significantly smaller numbers of terminally differentiated effector memory cells re-expressing CD45RA (TEMRA cells). Moreover, LN CD8+ T-cells strongly expressed the activation marker CD69, yet presented with higher levels of PD-1 compared to PB CD8+ T-cells. In line with high PD-1 expression, LN CD8+ T-cells exhibited a significantly lower production of IFNγ and TNFα, and a substantially reduced cytotoxic capability as indicated by lower GzmB production compared to PB T-cells. TEM cells in CLL patients' LNs exhibited different levels of PD-1 expression, and we observed a significantly higher percentage of cells with high PD-1 expression (PD-1hi) in LNs compared to PB, which was mainly composed of cells with intermediate PD-1 expression (PD-1int), (Figure 1). Comparison of PD-1hi and PD-1int subsets in LNs showed that PD-1hi cells expressed higher levels of CD69 compared to PD-1int cells, but were substantially inferior in GzmB production. Furthermore, PD-1hi cells expressed significantly higher levels of EOMES, a transcription factor that is known to drive terminal differentiation and exhaustion of T-cells in chronic infections. In contrast, PD-1int cells showed higher expression of the transcription factors T-BET and TCF7, which mediate long-term endurance of T-cells in chronic situations.
In summary, this study shows that exhausted T-cells are enriched in LNs rather than PB of CLL patients. Our data suggests that the increased leukemia-associated activation of CD8+ T-cells in patients' LNs drives the accumulation of PD-1hi exhausted T-cells. A better understanding of these processes will be essential for the development of novel immunotherapeutic approaches for CLL.
Figure 1:(A) CD8+ T-cells from paired peripheral blood (PB) and lymph node (LN) samples of CLL patients (n=7) were analyzed by flow cytometry for the expression of PD-1 (representative of seven samples). (B) The relative abundance of PD-1low, PD-1int and PD-1hi subsets within the CD45RO+ CCR7- CD8+ effector memory (EM) population in PB and LN was quantified.
Stilgenbauer: AbbVie: Consultancy, Honoraria, Research Funding; Genzyme: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Mundipharma: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Boehringer-Ingelheim: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal